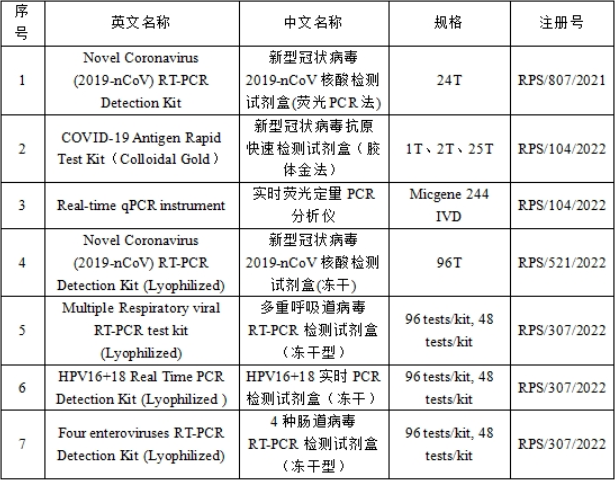
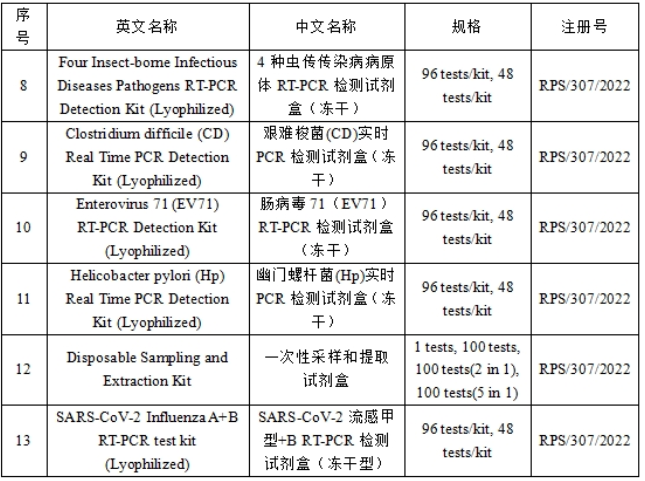
Rt-pcr detection kit for multiple respiratory viruses (lyophilized type), real-time PCR detection kit for HPV16+18 (lyophilized type), RT-PCR detection kit for 4 enterovirus (lyophilized type), RT-PCR detection kit for 4 pathogens of insect borne infectious diseases (lyophilized type), real-time PCR detection kit for Clostridium difficile (CD) (lyophilized type), intestine Virus 71 (EV71) RT-PCR detection kit (lyophilized), Helicobacter pylori (Hp) real-time PCR detection kit (lyophilized), one-time sampling and extraction Kit, SARS-CoV-2 influenza A +B RT-PCR detection kit (lyophilized)

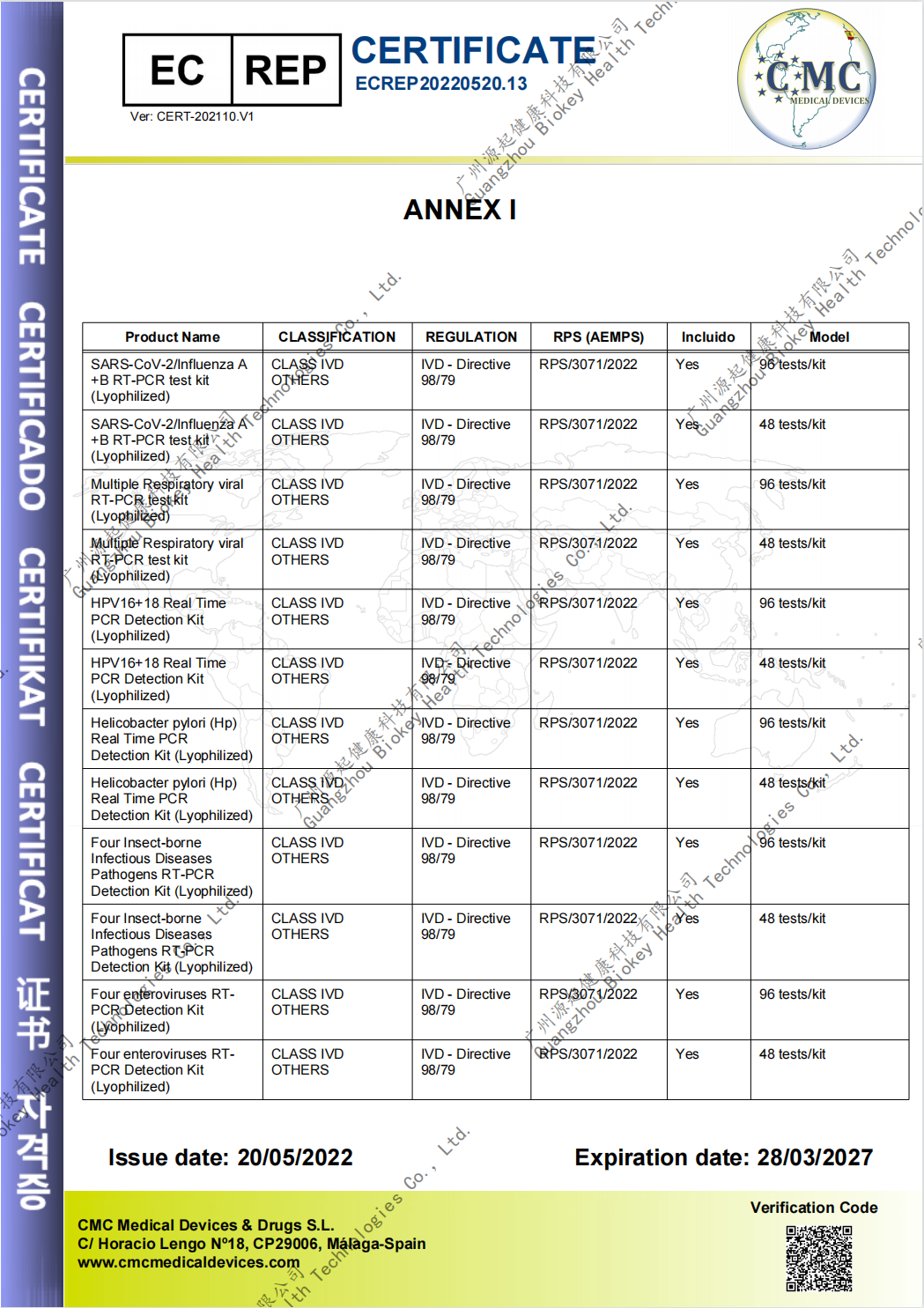

The "CE" mark is a safety certification mark and is regarded as the manufacturer's passport to open and enter the European market.
In the EU market, the "CE" mark is a mandatory certification mark. Products produced by enterprises within the EU or by other countries must be affixed with the "CE" mark if they want to circulate freely in the EU market. The process of obtaining a CE mark is often referred to as CE certification.