BIOKEY coronavirus nucleic acid detection reagent entered the Ministry of Commerce export "white list"
Date of publication:2022-09-28 Views:1952
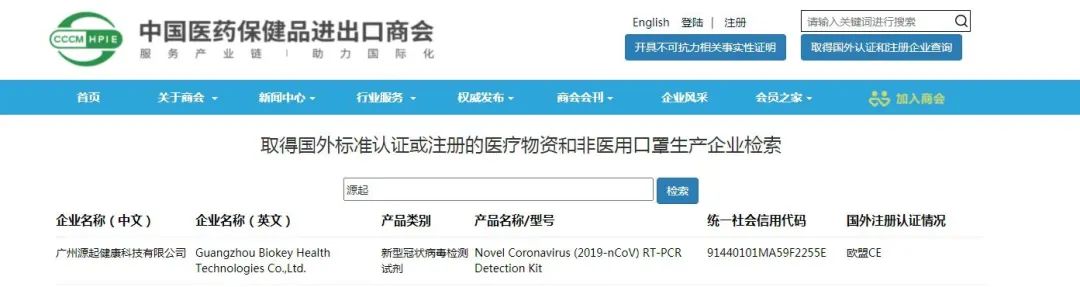
On April 25, 2020, the Ministry of Commerce, the General Administration of Customs and the State Administration for Market Regulation issued a notice on further strengthening the quality supervision of the export of epidemic prevention materials: Starting from April 26, export enterprises whose products have been certified or registered in foreign standards for COVID-19 testing reagents, medical masks, medical protective suits, ventilators and infrared thermometers are required to submit a written declaration at customs declaration, promising that the products meet the quality standards and safety requirements of the importing country (region). The customs shall check and release the products on the basis of the list of manufacturers certified or registered by foreign standards provided by the Ministry of Commerce (updated dynamically on the website of China Chamber of Commerce for Import and Export of Medicine and Health Products www.cccmhpie.org.cn, which is known as the export "white list" in the medical industry).
In a new global outbreak continues to spread the special period, for more effective support to the international community to jointly cope with global public crisis, after its strict product testing and audit check, medicine and health products import and export chamber of commerce in China recently updated the foreign standards certification or registration of medical supplies production enterprise listing "Biokey one of its top.