Recently, Biokey novel coronavirus antigen rapid detection kit (colloidal gold method) was included in the List of Medical Supplies Manufacturers with foreign standard Certification or Registration provided by the Ministry of Commerce. It meets the requirements of the Announcement on Further Strengthening the Quality Control of Export of Epidemic Prevention Materials No. 12, 2020 issued by the Ministry of Commerce, the General Administration of Customs and the State Administration for Market Regulation, and is qualified for overseas export sales. The overseas sales of Biokey's COVID-19 antigen testing reagents have been carried out in an orderly manner.
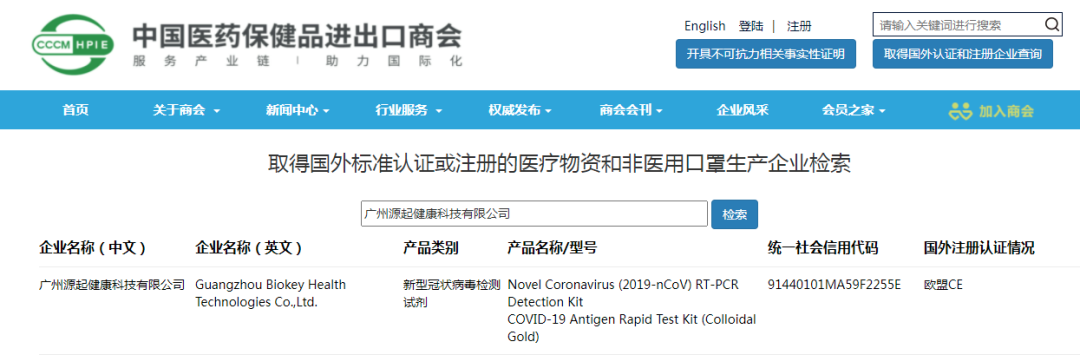
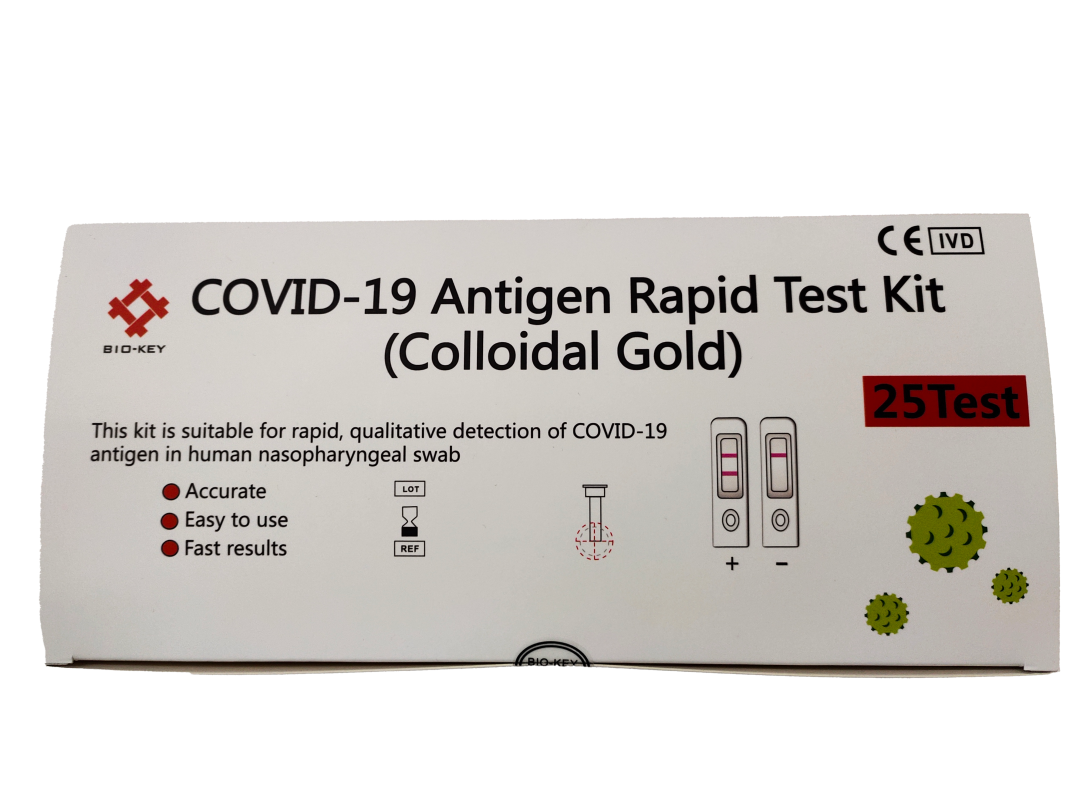
The novel coronavirus antigen detection reagent is 2019-NCOV novel coronavirus antigen detection reagent (colloidal gold method), which is used for rapid and qualitative detection of novel coronavirus antigens in human nasopharyngeal swab and oropharyngeal swab samples in vitro, which is helpful for the auxiliary examination of diseases caused by novel coronavirus infection.
Due to its rapid and convenient characteristics, the novel coronavirus antigen detection product can be used for rapid auxiliary diagnosis of suspected patients and rapid auxiliary screening of key populations, so as to promote early diagnosis and timely intervention of patients. It can better meet the needs of rapid on-site testing and prevention and control of the epidemic in various countries, and help the prevention and control of the novel coronavirus epidemic globally.
